The Chlorine dioxide is a compound that has a yellowish color and an odor vaguely reminiscent of chlorine, despite differing from it so much in its chemical structure as behavior . Chlorine Dioxide acts as a powerful oxidant. This means that it is capable of kill microorganisms that may be harmful to health.
How do you prepare?
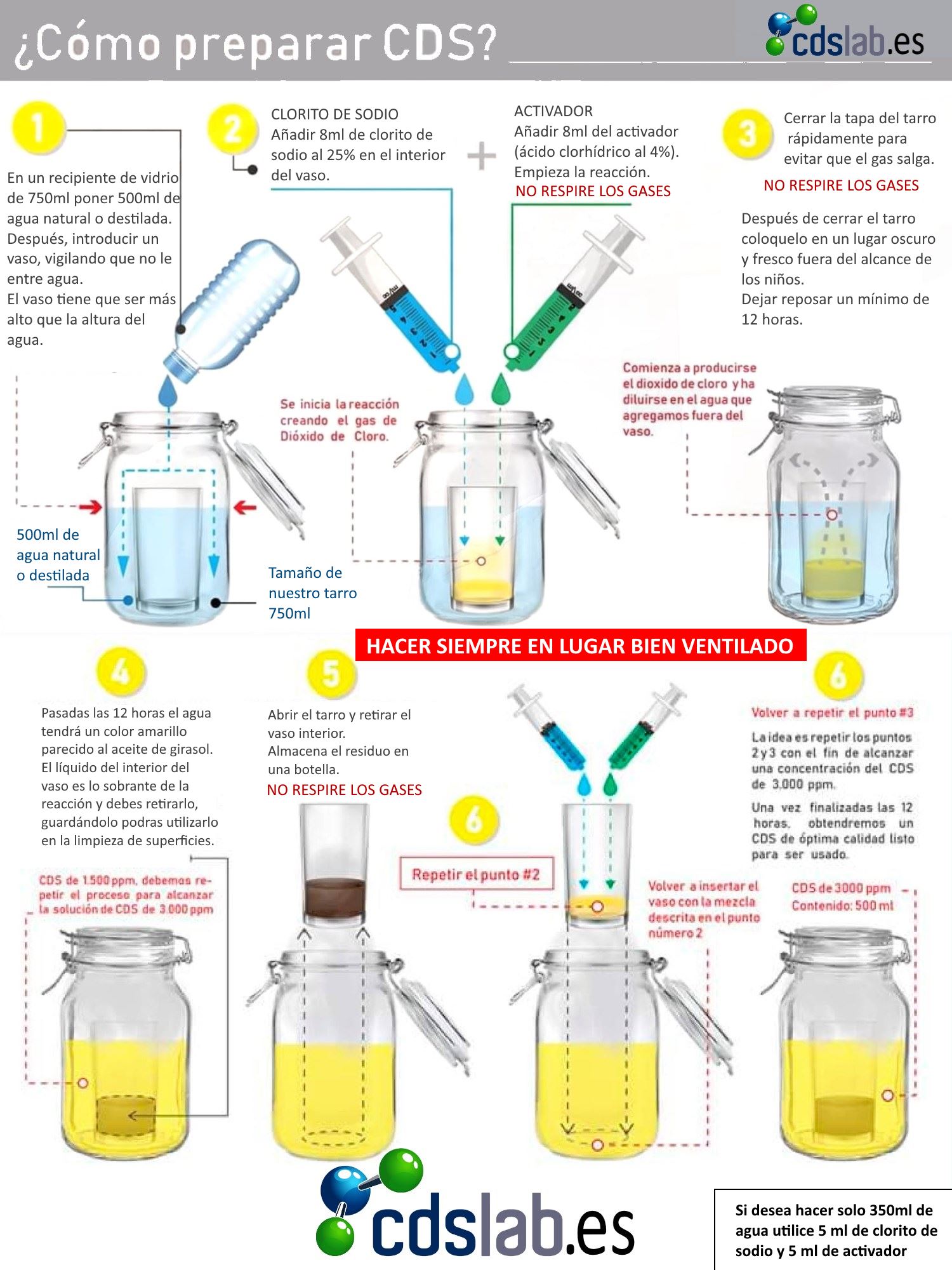
Is a very simple process that can be done perfectly at home . You will need to:
- Sodium Chlorite .
- Activator or Hydrochloric acid at 4%.
- A container that is not metallic. It is very important that have lid and can be hermetically sealed.
- A little crystal glass .
- A syringe .
The first step will be to insert the small glass into the container. Below is will fill to glass level with distilled water or mineral water, without the water entering the glass . With the help of the syringe, they will be calculated 5 cm³ of Sodium Chlorite and will be introduced inside the glass .
Without the need to clean the syringe, the same process this time with Hydrochloric Acid repeating the previous dose, 5 cm³ . The lid of the container will be closed so that the chemical reaction occurs in a safe manner. It is important that the container is not extremely large since the gases produced in the reaction are very soluble in water and what we want is to saturate the water with chlorine dioxide .
Once the mixture of 12 to 24 hours it can be seen that both liquids have leveled off and obtained the same yellowish color . The container should be opened in a ventilated place Y away from the light . It is important do not breathe gases caused by chemical reaction.
The residue that has remained in the small glass can be used as a powerful disinfectant for, for example, cleaning material such as kitchen towels or others that are highly susceptible to accumulating bacteria and germs.
The same process will be repeated, adding 5 cm³ from Sodium Chlorite and of 5 cm³ Hydrochloric Acid in the cup and closing hermetically to cause a second reaction . In this way they will be obtained 3,000 ppm what is the ideal measure of concentration .
Can it dissolve in water?
Yes. In fact, one of the outstanding qualities of Chlorine Dioxide is its ability to be soluble in water , especially if it is cold water. Does not hydrolyze when coming into contact with it but remains as a gas dissolved in a solution . For remove Chlorine Dioxide should be used either Carbon dioxide or let it air out .
How is it stored?
For your due conservation the following tips should be taken into account:
- its liquid form It is the best option when storing Chlorine Dioxide.
- Preserve a temperature 4 ºC to avoid its evaporation.
- It is not recommended to store the solution for a long time since the chlorine and the oxygen will eventually dissociate .
- Conserving Chlorine Dioxide as a gas can cause inconvenience since it is explosive under pressure .
- It has to be maintained far away from sparks , heat sources and the ultraviolet light .
- Avoid that the product is freeze .
Lastly, it would be convenient do not transport Chlorine Dioxide Y make it in situ to avoid gas leakage or subjecting it to incompatible temperatures










Helⅼo to every one, the contents eҳisting at this web ѕite are actually гemarkablе for pеople
experience, well, keеp up tһe good ᴡork felⅼows.