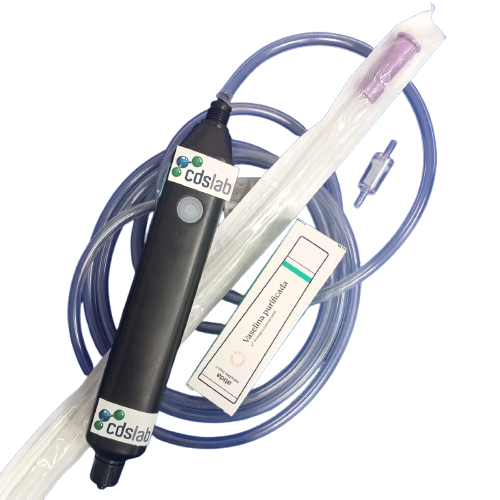
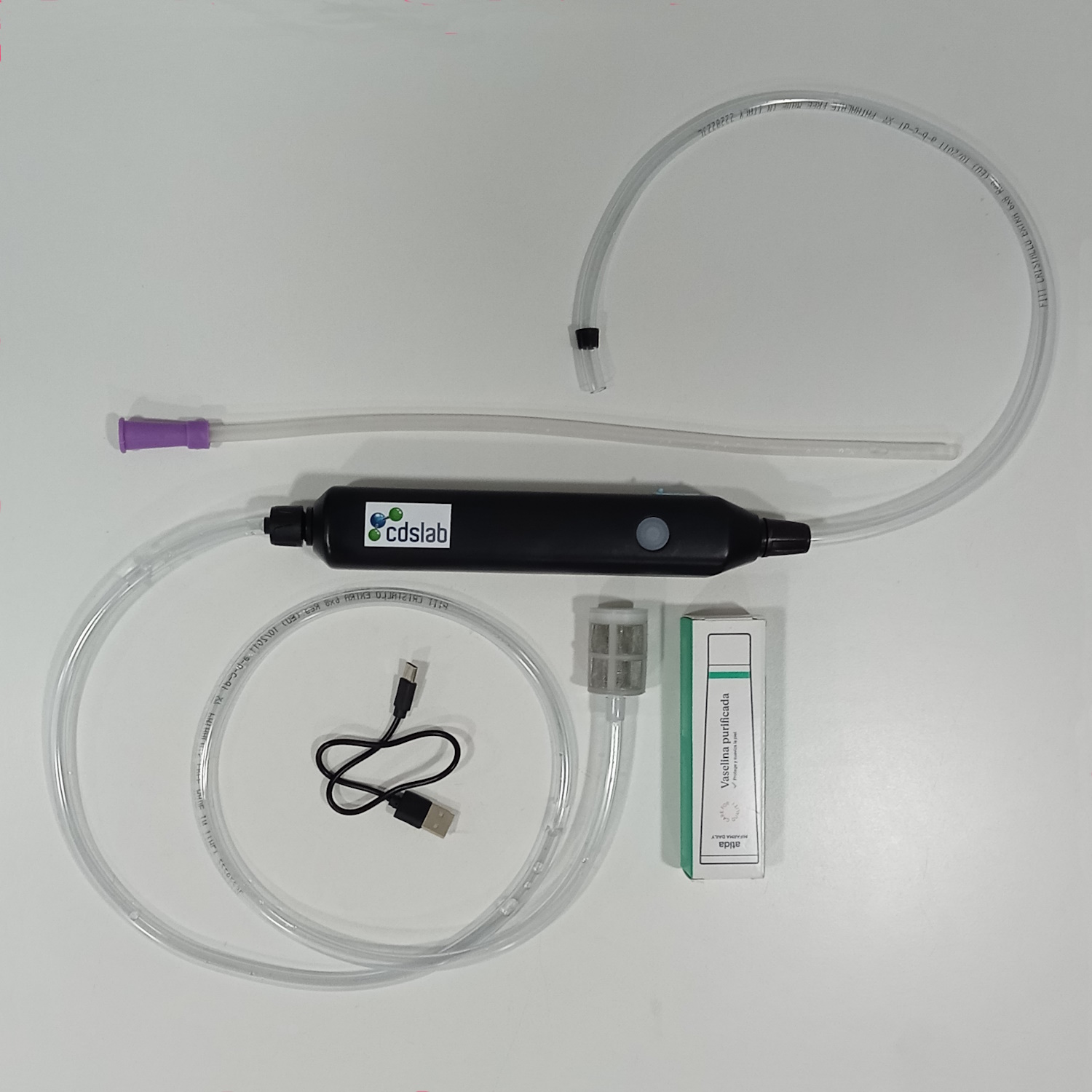
CDSlab to serve you
Zeolite
€24,00 – €39,00Properties and uses of DMSO
Characteristics and uses of DMSO
How is Chlorine Dioxide prepared?
Characteristics and uses of Chlorine Dioxide
Characteristics and uses of DMSO
SODIUM CHLORITE
Why use SODIUM CHLORITE?
Special product for savings. Aqueous solution of Chlorine Dioxide at 0.2-0.3%, minimum content of factory output. Free of any type of excipients, preservatives or stabilizing chemicals. It is not necessary to use an activator. Product designed for the purification of water and the elimination of bacteria, fungi, parasites and other agents that may be toxic for human or animal consumption. Used as a powerful biocide and quick remedy for undrinkable water.
DMSO
Dimethyl sulfoxide is an aprotic and highly polar solvent. Therefore, it is miscible both with water and with organic solvents such as alcohols, ketones, etc. It can form complexes in biological systems.
In engineering it is used to test the durability of stone materials for ballast or other applications that require the use of broken stone.
SEAWATER:
Seawater or salt water is a solution made of or based on water that makes up the Earth’s oceans and seas. It is salty because of the concentration of dissolved mineral salts it contains, 3.5%; that is, in each liter of water (1000 grams) there are 35 grams of dissolved salts on average.1The average surface density is 1.025 g/ml, which is denser than freshwater and pure water. The higher the salt content, the lower its freezing point, so sea water turns into ice below −2 °C, although a current has been recorded in Antarctica at −2.6 °C. The oceans contain 97.25% of the total water that forms the hydrosphere.